Biosimilars work, at a fraction of the cost
- Recognize that biosimilars as safe and effective as their originator biologic.
- Reassure patients that biosimilars work as well as the originator biologic
- Increase confidence in switching between biologics and biosimilars.
Biologic medications, such as monoclonal antibodies, are increasingly used to treat many diseases. Due to their high cost, biologics now account for over half of medication spending in the US.1 Biosimilars are less costly than original brand-name biologics and are now available for more than a dozen medications. However, biosimilars are not used, accounting for only account for 1 in 5 prescriptions.2 This module breaks through the noise to uncover the evidence about biosimilars, allowing prescribers to be more confident and patients to be reassured that biosimilars are just as safe and effective as the corresponding originator biologics.
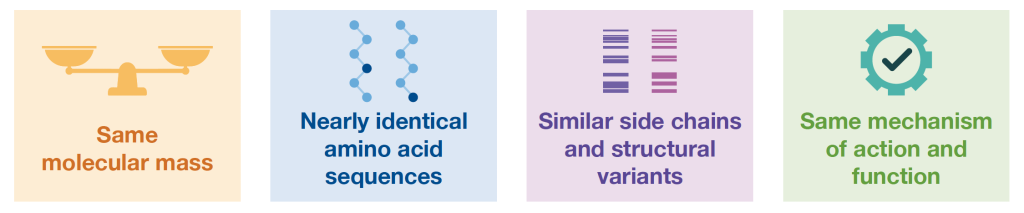
Biosimilars are highly similar to the originator
The FDA requires rigorous proof of similarity between biosimilars and their orignator.3 The areas of similarity include:
Biosimilars are just as safe and effective
Biosimilars are tested in head-to-head, randomized, controlled trials to establish efficacy and safety compared to originator biologics.
Patients can safely be switched to biosimilars
There is substantial data from randomized switching studies and real-world evidence that switching from biologics to biosimilars does not lead to any problems with safety, efficacy, and immunogenicity.4
Ensuring that clinicians and patients are comfortable with the evidence around biosimilars is important as biosimilars are increase relied on as more affordable options for patients.
Click here to contact us for more information about Alosa’s full academic detailing package.